Abstract
BACKGROUND. Bortezomib-melphalan-prednisone (VMP) and lenalidomide-dexamethasone (Rd) have represented standards of care for transplant-ineligible (NTE) newly diagnosed multiple myeloma (NDMM) patients (pts) until the recent introduction of daratumumab (Dara) in the frontline setting. However, no prospective randomized trial has directly compared VMP with Rd. Moreover, real-life older NTE pts are underrepresented in clinical trials, and only few data on safety and efficacy in this population are available.
AIMS. We conducted a randomized multicenter phase IV trial (NCT03829371; funded by the Italian Medicine Agency AIFA - Independent Research) to compare safety and efficacy of the two standard regimens, VMP vs Rd, in an unselected real-life population of NTE NDMM pts.
METHODS. NDMM pts ineligible for transplant due to age ≥65 years or comorbidities were randomized 1:1 to: 9 VMP cycles (42-day cycles, V: 1.3 mg/m2, days (dd) 1, 4, 8, 11, 22, 25, 29, and 32, cycles 1-4 and dd 1, 8, 22, and 29, cycles 5-9; M: 9 mg/m2, dd 1-4; P: 60 mg/m2, dd 1-4) vs continuous Rd (28-day cycles, R: 25 mg, dd 1-21; d: 40 mg, dd 1, 8, 15, and 22) according to standard practice. Pts gave written informed consent and were enrolled regardless of performance status, comorbidities, renal function, or baseline laboratory values. Pts were stratified according to the International Myeloma Working Group (IMWG) frailty score and cytogenetic risk by fluorescence in situ hybridization [high risk if positive for del(17p), t(14;16), or t(4;14)]. The primary endpoint was progression-free survival (PFS) in the intention-to-treat (ITT) population. Key secondary endpoints included response rates, overall survival (OS), and safety.
RESULTS. At the data cut-off (July 4, 2022), 231 pts were randomly allocated to receive VMP (n=114) or Rd (n=117). Baseline characteristics were evenly balanced between the two arms: median age was 77 (IQR 73-80) and 76 years (IQR 73-79); 49% vs 50% of pts were frail; 17% vs 19% pts had high-risk cytogenetics, respectively.
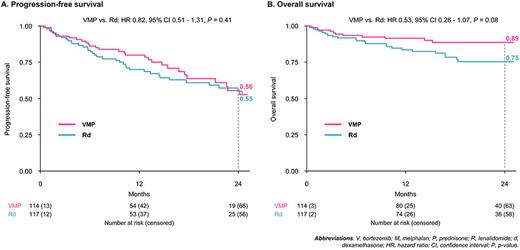
After a median follow-up of 19 months, the median PFS in the ITT population was 29.6 vs 26.2 months with VMP vs Rd (hazard ratio [HR] 0.82, 95% CI 0.51-1.31, P=0.41; Figure - panel A). However, the HR was not constant over time: from 0.65 (95% CI 0.36-1.19, P=0.16) during the first year of treatment (suggesting a slight benefit of VMP vs Rd) to 1.37 (95% CI 0.65-2.86, P=0.40) after the first year of treatment (suggesting a slight benefit of continuous Rd therapy).
The subgroup analysis according to cytogenetic risk showed a significant effect modification of the comparison VMP vs Rd in terms of PFS, with an HR=0.21 (95% CI 0.04-0.99) in the high-risk group vs an HR=1.24 (95% CI 0.70-2.18) in the standard-risk group (interaction P=0.036). No significant effect modification was observed in terms of age or frailty status.
In the ITT population, the 2-year OS was 89% with VMP vs 75% with Rd (HR 0.53, 95% CI 0.26-1.07, P=0.08; Figure - panel B).
No safety concerns were reported. The most frequent grade (G)3-4 adverse events (AEs) with VMP were thrombocytopenia (15%) and neuropathy (7%), while neutropenia (23%), infections (12%), and dermatologic toxicities (9%) were the most frequent G3-4 AEs observed in the Rd arm.
Sixty-six percent of pts in the VMP and 59% in the Rd arms had ≥1 dose reductions of any drug, including 35% of pts in the VMP arm switching to once-weekly V before cycle 5.
CONCLUSION. We confirmed the efficacy of VMP and Rd in an older real-life population of NTE NDMM pts including ~50% of frail pts. VMP and Rd were equally effective in standard-risk pts and in all frailty subgroups in terms of PFS. However, high-risk pts benefited more from VMP (HR 0.21), suggesting that the doublet Rd is suboptimal in this subgroup. Safety data were consistent with those previously reported. Of note, only one third of real-life pts were able to receive full-dose VMP or Rd therapy according to their schedule. Despite the limited follow-up, the difference in terms of OS between the two arms may be explained by the poor outcomes of patients failing lenalidomide-based treatments.
Importantly, following a recent amendment, as of July 2022, enrolled pts will be randomized to Dara-VMP vs Dara-Rd. Decision-making and treatment selection will be further supported by a future analysis of this addition of Dara, based on patient and disease characteristics.
Disclosures
Bringhen:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy. D'Agostino:GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures; Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures. Giuliani:Millennium Pharmaceuticals: Other: Sponsor of clinical trials; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Other: Sponsor of clinical trials, Support for conferences, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Support for conferences, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees. Zambello:Amgen: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Rossi:Servier: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Galieni:abbvie: Membership on an entity's Board of Directors or advisory committees; takeda: Membership on an entity's Board of Directors or advisory committees. Mannina:GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees. Patriarca:GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Cavo:Janssen: Honoraria, Speakers Bureau; AbbVie, Amgen, Bristol Myers Squibb/Celgene, Pfizer, GlaxoSmithKline, Sanofi, Roche, Takeda: Consultancy, Honoraria. Mangiacavalli:Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Participation in meetings; Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: Participation in meetings; Johnson & Johnson: Membership on an entity's Board of Directors or advisory committees, Other: Participation in meetings; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Other: Participation in meetings; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Other: Participation in meetings; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Participation in meetings. Benevolo:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Boccadoro:GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Mundipharma: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; AbbVie: Honoraria. Larocca:Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal